- HOME
- SEPAREL Technology
What is Degasification & Aeration?
The removal of gas that is dissolved in liquid.
Liquids that contact air contain dissolved gasses that are not visible, such as N2 and O2. These dissolved gasses cause various problems, including the corrosion of pipes.
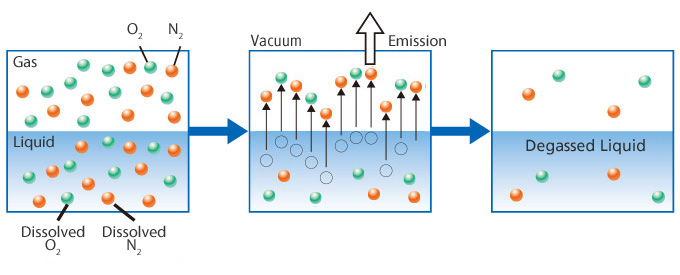
Visible bubbles are caused by dissolved gas. When the temperature increases or pressure drops, gas bubbles appear in liquids. Problems related to these bubbles can be prevented by degasification.
- ※For further information on usage, please visit the Applications page.
Dissolving gases into liquid.
Aeration is the opposite of degasification and it occurs when gas is dissolved into liquid.
A specific gas, with an intended function, can be added to a liquid via aeration.
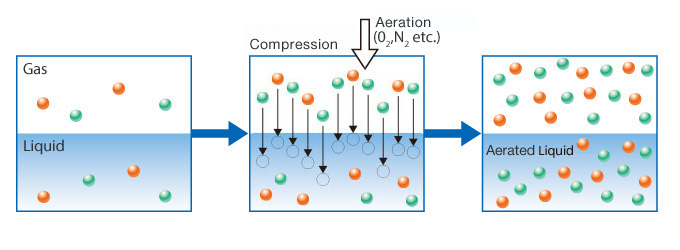
For example, carbonated water (sparkling water) is produced by aerating water with CO2.
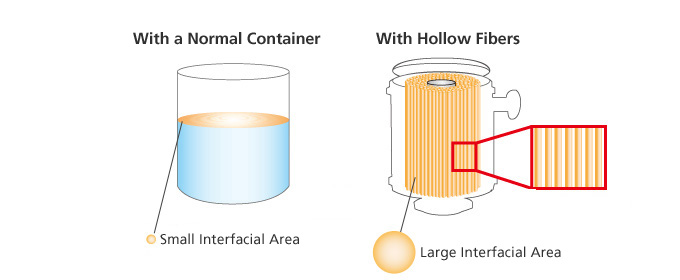
A larger interfacial area between a gas and liquid is most effective.
Basic Principles
According to Henry’s Law, degasification performance depends upon the interfacial area between gas and liquid. When the partial pressure of gas decreases, the partial pressure of gas dissolved in liquid also goes down. This mean that, if the pressure of gas is set to “0” (as absolute pressure), the volume of gas dissolved in liquid also becomes “0.”
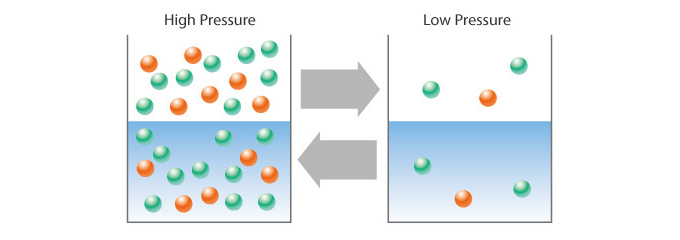
How Do We Improve Performance?
The larger the interfacial area, the more effective degasification and aeration performance becomes. However, enlarging the interfacial area typically requires a lot more space. Also, degasification and aeration would take longer to occur and, in turn, possibly change a liquid’s composition.
Through the use of a proprietary hollow fiber membrane, SEPAREL modules effectively meet all degasification and aeration needs.
Decompression Method
For more information, please visit.
"Basic Principles of Degasification & Aeration."
Heating Method
Increase the liquid temperature to decrease solubility and, therefore, remove gases.
Disadvantage
- Liquid composition may change.
Chemical Method
Remove dissolved gases via chemical reaction.
Disadvantage
- Applicable only for some specific gases.

![]() Tank System
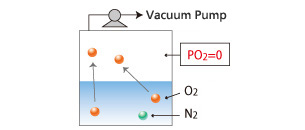
Tank System
The vacuum inside of the tank removes dissolved gases.
【Disadvantage】
- Because the interfacial area is small, degasification takes longer.
![]() Vacuum Degasification
Vacuum Degasification
Degasification from a sprayed liquid.
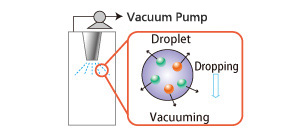
- Microparticulation increases surface area, which increases the interfacial area between gas and liquid.
- This method can also be adapted for liquids contaminated with certain particles.
【Disadvantages】
- Higher installation cost.
- Large equipment necessary.
【Advantages of Using a Hollow Fiber Membrane】
- A larger interfacial area
- Gas diffusion facilitated by flowing liquid
- In-line Degasification
- Low installation cost
- Can combine with a nitrogen gas displacement system
DIC’s Membrane Degasification System
DIC’s propriety membrane is composed of straw-shaped hollow fibers. Degasification is performed in and outside of the fibers.
- ※For more information on DIC’s hollow fiber membrane, please click here.
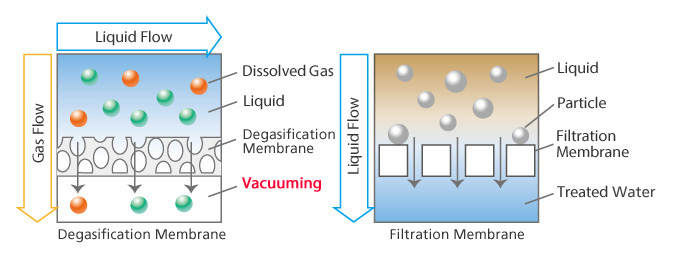
DIC Degasification Membrane
- DIC’s proprietary non-porous membrane technology. (No holes)
- Degasification occurs because only gas can pass through the membrane, not liquid.
Filtration Membrane
- Porous membrane with holes.
- Liquid will pass through with gas while only larger fungi particles are blocked.